Written By Dr. Wesley Cheung, DVM
Ringworm remains a challenging condition to manage in shelter dogs and cats, due to its long incubation period, risk of re-infection, limitations in diagnostic test options, long and frustrating treatment course, and zoonotic potential. Effective diagnosis and management are important to minimize unnecessary treatments, prolonged stays, and strain on shelter operational resources.
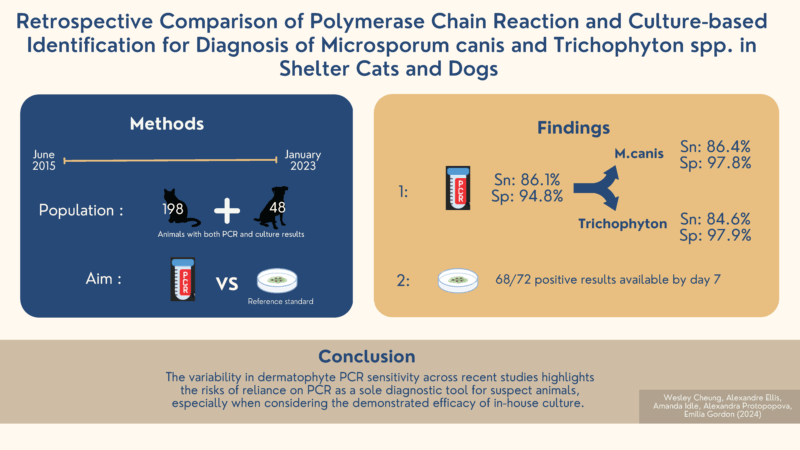
In our recent retrospective study, we compared polymerase chain reaction (PCR) and culture-based identification methods for the diagnosis of Microsporum canis and Trichophyton spp. in shelter dogs and cats.
Our study included data from the BC SPCA, consisting of 34 shelter locations, from June 2015 to January 2023. As part of the shelter intake protocol, all cats and dogs were screened with a Wood’s Lamp and examined by trained staff members. In-house culture and external laboratory PCR results were available for 246 cats and dogs with suspicious lesions.
Fungal cultures were performed at one central shelter location, using DTM/ESA culture plates. Culture plates were examined every one to three days for 14 days, and results were interpreted using a modified version of the P-scoring system. The P-scoring system is a semi-quantitative method to assess the number of colony-forming units (cfu) on a dermatophyte culture plate.1 At BC SPCA, the P-scoring system was used to differentiate:
- Cats that were truly infected;
- Cats that may have picked up ringworm spores on their coats but were not truly infected, also called “dust mops”; or
- Cats that were likely not infected nor “dust mops”.
The ringworm species growing on each plate was identified by microscopy and recorded alongside P-scores. Based on the P-scores, patients were designated as either “positive” or “negative”.
The main findings of our study were that, when using culture as the reference standard:
- PCR had a sensitivity of 86.4% for Microsporum canis and 84.6% for Trichophyton spp.;
- PCR had a specificity of 97.9% for Microsporum canis and 97.7% for Trichophyton spp.;
- All animals with a negative PCR and positive culture result, which were considered in this study to be “false negative” PCR results, had P3 score on fungal cultures (10 or more cfu per culture plate); and
- 94.4% (68/72) of “positive” culture results were confirmed within 7 days of plating. This short duration was consistent with the findings of previous research.
Using in-house culture plates, staff were able to quickly identify most infected cats within 7 days. The P-scoring system allowed for the differentiation of “dust mop” cats, which do not require the same intensive treatment. PCR cannot differentiate between “dust mop” and truly infected cats.
The variability in ringworm PCR sensitivity across recent studies emphasises the risk of relying on PCR as a sole diagnostic tool for suspect animals.2 In our study, 14 of every 100 infected animals would be missed on PCR alone, so a negative result in a suspect animal should be interpreted cautiously. However, our results suggest that PCR is an excellent tool for confirming truly infected animals, so that they can be treated and adopted out efficiently.
References
- Moriello KA, Coyner K, Paterson S, Mignon B. Diagnosis and treatment of dermatophytosis in dogs and cats.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017;28(3):266-e68.
- Frost K, Schick A, Mount R. A retrospective analysis of the concordance of in‐house fungal culture and a commercial quantitative PCR from 16 dermatology referral practices across the USA (2018–2019). Vet Dermatol. Published online June 6, 2022:vde.13085.
